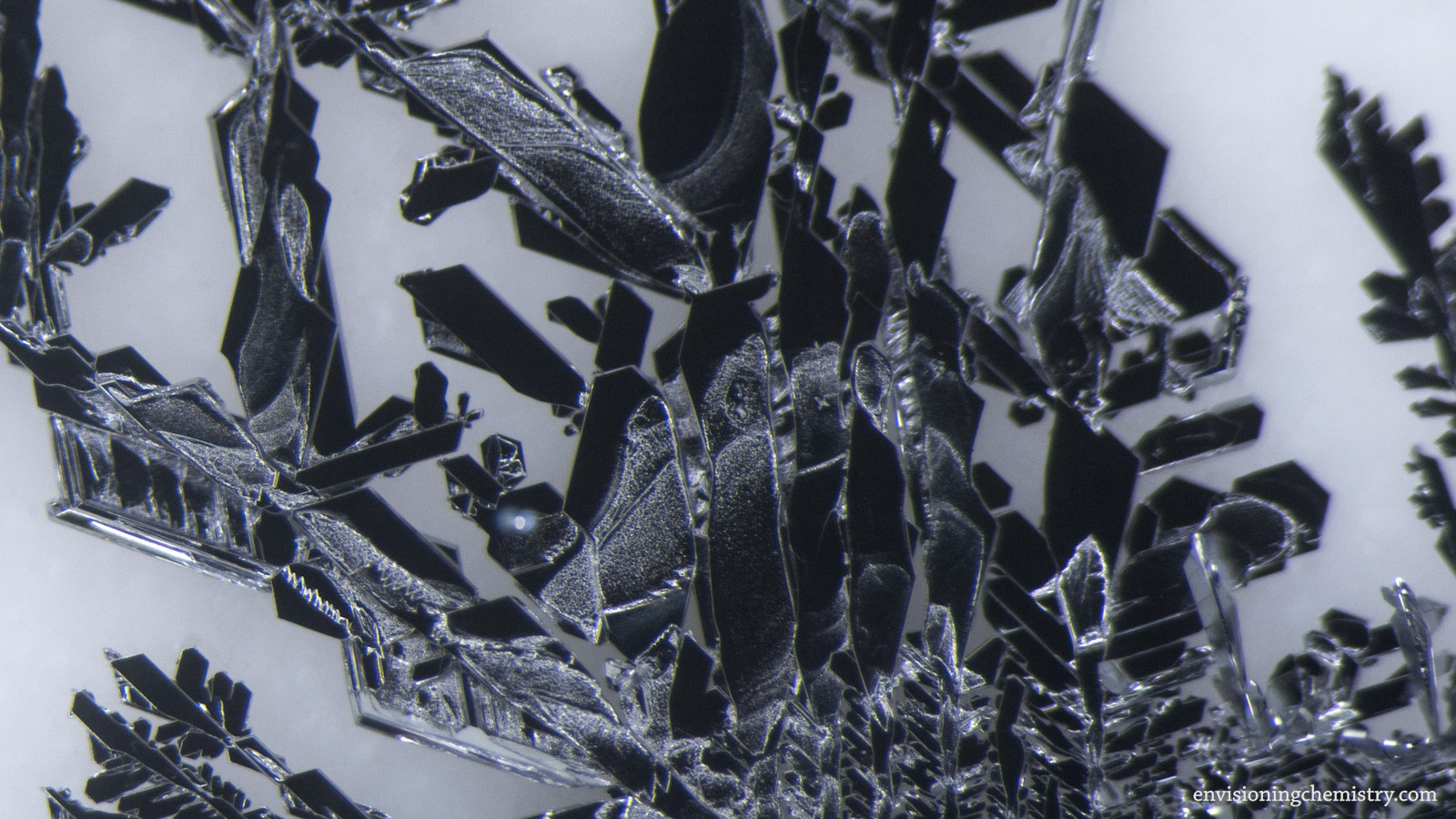
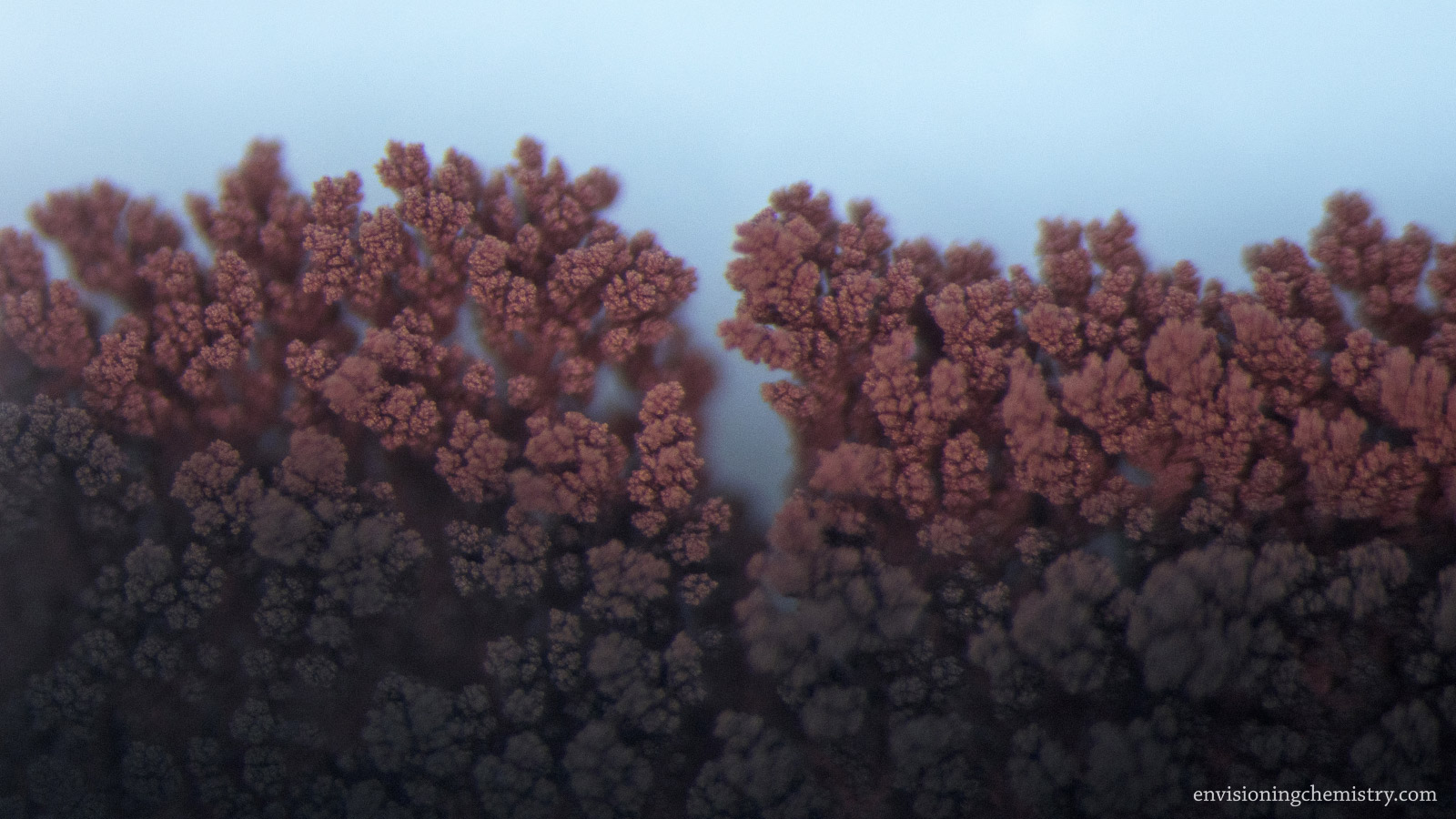
Metal Displacement II
A metal can displace a less reactive metal from its salt solution. Zinc is more reactive than silver, lead, copper, and tin. As a result, when pieces of zinc metal were dropped in the solutions of silver nitrate, lead(II) nitrate, copper(II) sulfate, and tin(II) chloride, microscopic metal crystals emerged on the surface of zinc and started to grow. In addition, copper was used for displaying silver as well. These growth processes were recorded with a macro lens and a microscope in this film.