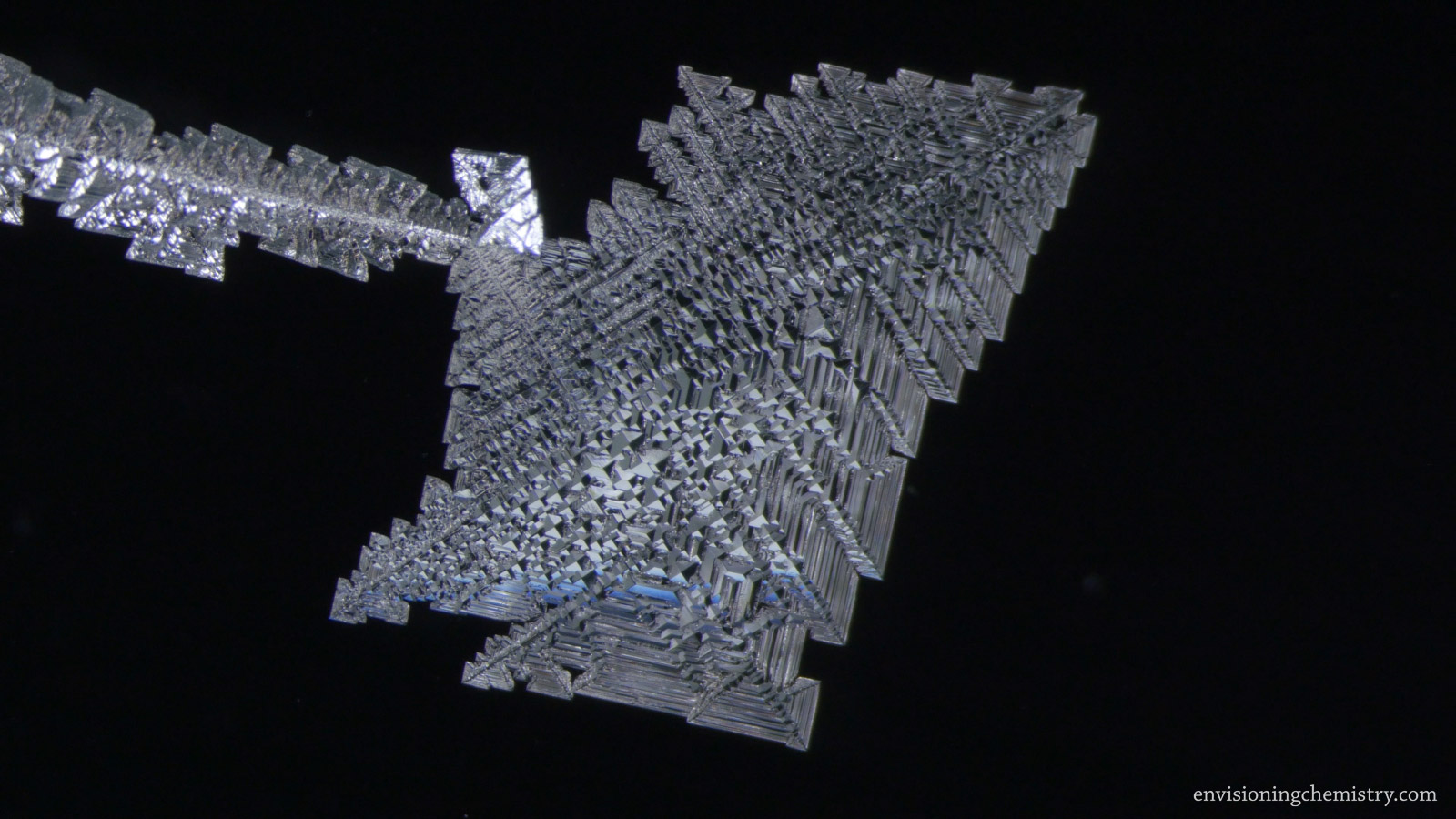
Electrodeposition
During electrodeposition, metal cations in a solution get reduced at the electrode connected to the negative terminal of a power supply. Beautiful metal structures can be generated in this process. In this film, the electrodeposition processes of 5 metals, including copper, tin, zinc, lead, and silver, were recorded under a microscope. If you think you know what metals look like, well, think again!